COVID-19: Novavax vaccine receives first emergency use authorization
Nov. 1, 2021
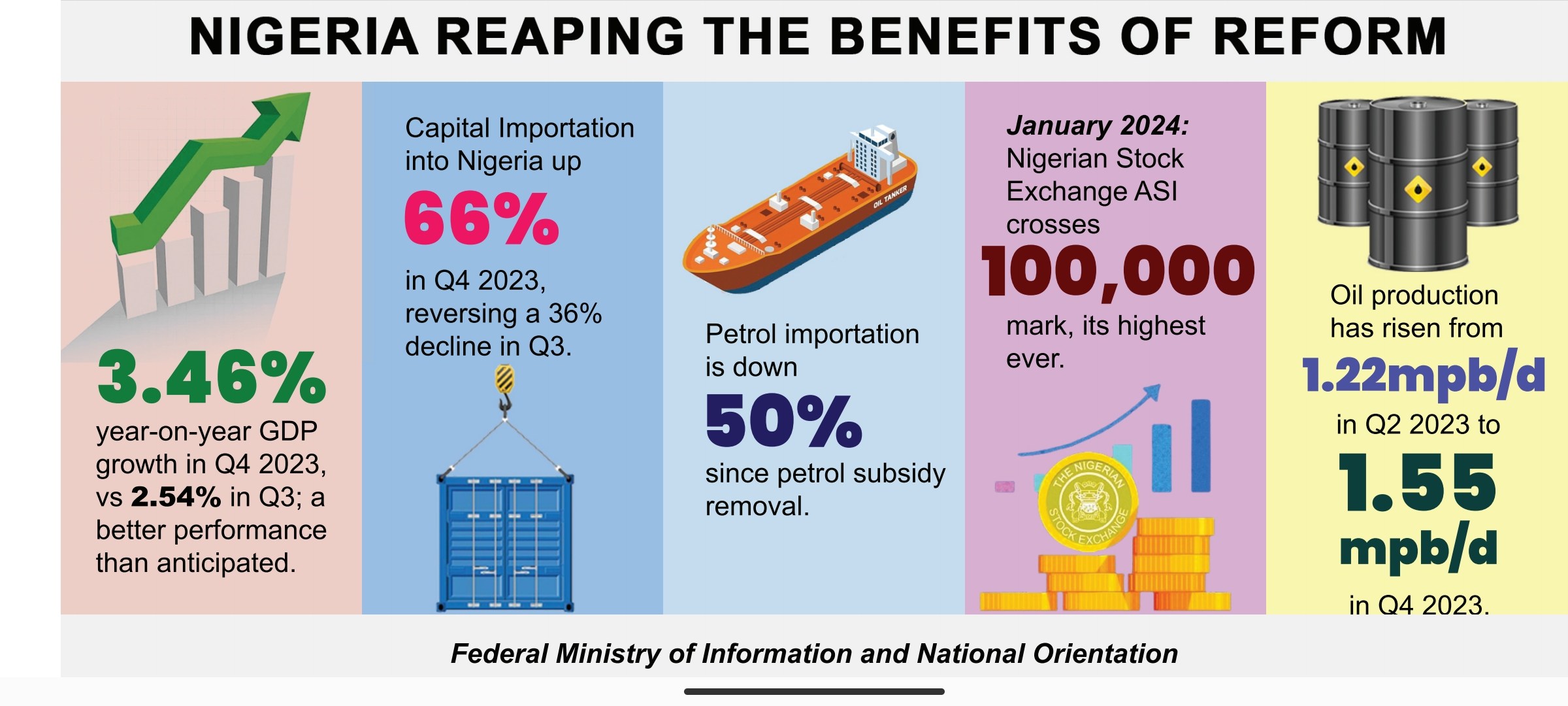
Novavax Inc and partner Serum Institute of India said on Monday they received emergency use authorization for their COVID-19 vaccine in Indonesia, making it the first approval anywhere in the world for Novavax.
Shares of Novavax rose about 11% before the opening bell after the company also said it had filed an application for emergency use for the vaccine to Canada and the European Medicines Agency.
For Indonesia, the shot will be manufactured by the world’s largest vaccine manufacturer, Serum Institute, and sold under the Indian company’s brand name, Covovax.
Novavax said initial shipments into Indonesia are expected to begin imminently.
The country is slated to receive 20 million doses of the protein-based vaccine this year, according to the government.
Penny Lukito, chief of the National Agency for Drug and Food Control of Indonesia, did not immediately respond to a Reuters request for comment.
The company, along with Japanese partner Takeda Pharmaceutical Co (4502.T), said on Friday it was preparing to seek regulatory approval for a rollout in Japan early next year. read more
Novavax and Serum Institute have committed to together provide more than 1.1 billion doses to the COVAX facility, co-led by the World Health Organization. The supply of the vaccine will start this year after securing an emergency use listing from the WHO and continue into 2022.
Novavax has delayed filing for U.S. approval to the end of this year, and Politico reported last month that the company has faced production and quality problems.
The Novavax shot was shown to be more than 90% effective, including against a variety of concerning variants of the coronavirus in a large, late-stage U.S.-based clinical trial.
REUTERS